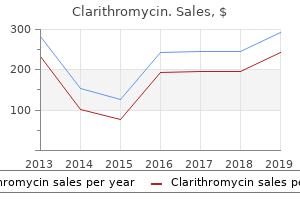
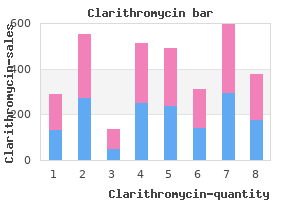
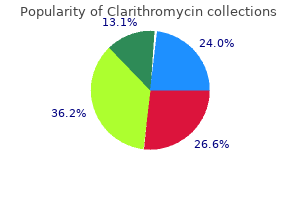
"Order clarithromycin cheap online, gastritis gi bleed".
By: X. Jaroll, M.S., Ph.D.
Medical Instructor, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo
In India gastritis relieved by eating order clarithromycin no prescription, the class of drugs called "branded generics" are in effect the same as branded products chronic gastritis outcome purchase clarithromycin 500mg free shipping. On the other hand gastritis diet 91352 purchase clarithromycin cheap online, Alerid of Cipla is a branded product since it is promoted to the medical profession gastritis symptoms causes buy cheap clarithromycin line. Trade margins for wholesale and retail are of the order of 10 and 20 percent respectively for brand name drugs like Alerid and in which the company spends a lot on promotion and marketing to the medical profession. All large Indian companies like Ranbaxy, Cipla, Zydus Cadila, Lupin, Alembic, et al, are involved in the sale of branded generics. Neither big pharma nor traditional generic companies are happy about the competition from branded generics, and both are mounting legal challenges. Branded generics are priced somewhere between brand-name drugs and true generics, and their market share is growing rapidly. They are likely to become very important in the biotech industry, where there are no traditional generics because it is difficult to show they are equivalent to the originals. Yes, definitely - from the point of view of consumers and patients but not according to the manufacturers. From the point of view of the consumer it is difficult to distinguish between similar sounding names of many brands (see Tables 1 and 2 below and Annexure 4). Since India till 2004 did not recognize product patents (for definition of product patents, etc. Very few brands (trade names) are actually registered with the trademark registry in Mumbai. As per Indian law, registered trade names (also called brands) are the exclusive property of the holder for a particular "class of products" such as medicines, vehicles, pens etc. Such registered trade names can be freely used for other classes such as Crocin for a pen or Maruti for paracetamol. Source: Vikas Adhyayan Kendra, Mumbai EssentialDrugs 105 low sodium salt marketed by Dabur and meant for hypertensives! Glaxo Smith Kline Pfizer Infar Novartis Emcure Pharma Novartis Pharma Orchid Unichem Ltd. Labs Vintage Labs Use Vitamin supplementation Antibiotic Worm infestation Pain, inflammation. A patient with arthritis may end with Vitamin C, a patient with depression may be dispensed an anti-allergic, an obese diabetic given metformin may be given gliclazide instead, as a result of these confusing brand names. Sometimes the same manufacturer markets drugs which are different but under similar sounding (and similar looking at first glance) brand names. Why is it felt in India that medicines marketed under brand names are of better quality than thosemarketedunderonlygenericnames? It is argued they are likely to be of better quality because companies that have invested in building a brand would not compromise with quality. However it is seen that some very wellknown drug companies and brands are frequently hauled up for violations of quality (see Chapter 4 for more details). There is no therapeutic difference between a medicine selling under a generic name and an equivalent drug selling under a brand name. The difference is in price, with generics selling normally much cheaper than brand name drugs. Should endusers and prescribers rely on the quality of medicines marketed under generic names? Schedule M certification is a must postJuly 2005 in India although there are still many companies, at the time of publication of this book, who are yet to fulfill Schedule M requirements. It does not mean a drug which is available in bulk packing, as contrasted to those in strips or blister packing, is necessarily of poor quality. Neither does it mean that a medicine which is very attractive packaged is necessarily of good quality. The best way to check for quality is through reputed quality control laboratories - reputed for integrity. The pharmacopoeias of different countries specify recommended quality standards and methods of analysis for selected pharmaceutical products, excipients, and dosage forms. There are also quality assurance guidelines in the areas of production, testing, and distribution of medicines.
Initially gastritis diet 2 go order 250 mg clarithromycin fast delivery, the committee decided to grade only the strength of the evidence chronic gastritis surgery purchase clarithromycin 250mg on line, using a 3-tier scale (table 1) used in a recent guideline from both societies [10] gastritis prognosis cheap clarithromycin line. Evidence from well-designed gastritis milk buy clarithromycin 250 mg without a prescription, controlled trials without randomization (including cohort, patient series, and case-control studies). In some instances, therapy recommendations come from antibiotic susceptibility data without clinical observations. The implication of a strong recommendation is that most patients should receive that intervention. Industrial models suggesting that variability per se is undesirable may not always be relevant to medicine [15]. For this reason, the committee members feel strongly that 100% compliance with guidelines is not the desired goal. However, the rationale for variation from a strongly recommended guideline should be apparent from the medical record. Conversely, moderate or weak recommendations suggest that, even if a majority would follow the recommended management, many practitioners may not. One document cannot cover all of the variable settings, unique hosts, or epidemiologic patterns that may dictate alternative management strategies, and physician judgment should always supersede guidelines. In addition, few of the recommendations have level I evidence to support them, and most are, therefore, legitimate topics for future research. Subsequent publication of studies documenting that care that deviates from guidelines results in better outcomes will stimulate revision of the guidelines. Although these guidelines are evidence based, the committee strongly urges that deviations from them not necessarily be considered substandard care, unless they are accompanied by evidence for worse outcomes in a studied population. Protocol design varies among studies, and the preferable randomized, parallel group design has been used in only a small minority. Confirmatory studies that use randomized, parallel groups with precisely defined treatments are still needed, but a consistent pattern of benefit is found in the other types of level I studies. Published protocols have varied in primary focus and comprehensiveness, and the corresponding benefits vary from one study to another. However, the most impressive aspect of this literature is the consistently beneficial effect seen in some clinically relevant parameter after the introduction of a protocol that increases compliance with published guidelines. A decrease in mortality with the introduction of guidelinebased protocols was found in several studies [19, 21]. A 5-year study of 28,700 patients with pneumonia who were admitted during implementation of a pneumonia guideline demonstrated that the crude 30-day mortality rate was 3. Lower mortality was seen in other studies, although the differences were not statistically significant [22, 23]. Studies that documented lower mortality emphasized increasing the number of patients receiving guideline-recommended antibiotics, confirming results of the multivariate analysis of a retrospective review [24]. When the focus of a guideline was hospitalization, the number of less ill patients admitted to the hospital was consistently found to be lower. However, patient satisfaction among outpatients was lower after implementation of this guideline, despite survey data that suggested most patients would prefer outpatient treatment [26]. Protocols using guidelines to decrease the duration of hospitalization have also been successful. A randomized, parallel group study introduced a pneumonia guideline in 20 of 36 small Oklahoma hospitals [29], with the identical protocol implemented in the remaining hospitals in a second phase. Serial measurement of key process measures showed significant improvement in time to first antibiotic dose and other variables, first in the initial 20 hospitals and later in the remaining 16 hospitals. The difficulty in implementing guidelines and changing physician behavior has also been documented [28, 33]. Clinically relevant outcome parameters should be evaluated to measure the effect of the local guideline. Just as it is important not to focus on one aspect of care, studying more than one outcome is also important. Other reasons for avoiding unnecessary admissions are that patients at low risk for death who are treated in the outpatient setting are able to resume normal activity sooner than those who are hospitalized, and 80% are reported to prefer outpatient therapy [26, 35]. No study has documented that simply changing 1 metric, such as time to first antibiotic dose, is associated with a decrease in mortality. Of these, rapid and appropriate empirical antibiotic therapy is consistently associated with improved outcome. We have also included elements of good care for general medical inpatients, such as early mobilization [30] and prophylaxis against thromboembolic disease [31].
Buy clarithromycin 500mg with visa. ULCER ANSWERS.
Ito Y gastritis bile reflux diet buy genuine clarithromycin line, Tomoda C gastritis english buy clarithromycin 250 mg visa, Uruno T gastritis child diet generic clarithromycin 500 mg, Takamura Y gastritis diet 91303 discount clarithromycin on line, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A 2006 Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. Weak Recommendation No Recommendation Balance of benefits and risks cannot be determined 373 Page 374 of 411 374 Table 2. Recommendations (for Therapeutic Interventions) based on strength of evidence* Thyroid Downloaded from online. The description of supporting evidence is different for diagnostic accuracy studies. Interpretation of the American Thyroid Association Guideline Grading System for Diagnostic Tests Recommendation Accuracy of Diagnostic Implications Information versus Risks and Burden of Testing* Knowledge of the Patients: In the case of an accurate Strong Recommendation diagnostic test result test for which benefits outweigh clearly outweighs risks risks/burden, most would want the and burden of testing or diagnostic to be offered (with vice versa appropriate counseling) a patient should request to discuss the test if it is not offered. In contrast, for a test in which risks and burden outweigh the benfits, most patients should not expect for the test to be offered. Clinicians: In the case of an accurate test for which benefits outweigh risks/burden, most patients should be offered the diagnostic test (and provided relevant counseling). Counseling about the test should include a discussion of the risks, benefits, and uncertainties related to testing (as applicable), as well as the implications of the test result. In contrast, for a test in which risks and burden outweigh the perceived benefits, most patients should not be offered the test, or if the test is discussed, the rationale against the test should, for the particular clinical situation, be explained. Policymakers: In the case of an accurate test for which benefits outweigh risks/burden, availability of the diagnostic test should be adopted in health policy. In contrast, for a test in which risks and burden outweigh the perceived benefits, some restrictions on circumstances for test use may need to be considered. Patients: most would want to be informed about the diagnostic test, but Thyroid Downloaded from online. Weak Recommendation Knowledge of the diagnostic test result 375 Page 376 of 411 376 closely balanced with risks and burden of testing some would not want to seriously consider undergoing the test a decision may depend on the individual circumstances. Clinicians: different choices will be appropriate for different patients, and counseling about the test (if being considered) should include a discussion of the risks, benefits, and uncertainties related to testing (as applicable), as well as the implications of the test result. Counseling the patient on why the test may be helpful or not, in her/his specific circumstance, may be very valuable in the decision-making process. Policymakers: policymaking decisions on availability of the test will require discussion and stakeholder involvement. Balance of knowledge of Decisions on the use of the test based the diagnostic test result on evidence from scientific studies cannot be determined cannot be made. No Recommendation * Frequently in these guidelines, the accuracy of the diagnosis of thyroid cancer (relative to a histologic gold standard) was the diagnostic outcome unless otherwise specified. However, prognostic, disease staging, or risk stratification studies were also included in the grading scheme of diagnostic studies. For disease staging systems, the implication for use would be on the part of the clinician, in reporting results in the medical record and communicating them to the patient (at applicable time point in disease or follow-up trajectory), as opposed to offering a specific choice of staging/risk stratification system to the patient. Recommendations (for Diagnostic Interventions) based on strength of evidence Recommendation and Evidence Quality Strong Recommendation High-quality evidence Thyroid Downloaded from online. Methodologic Quality of Supporting Evidence Interpretation Moderate-quality evidence Low-quality evidence Evidence from one or more well-designed non-randomized diagnostic accuracy studies. Insufficient Insufficient evidence to recommend for or against routinely offering the diagnostic test. Item R1* R2 R3 R4 R5* R6 R7 R8** F1**, F2**, T6** R9**, F1**, T7** R10 R11 R12 R13-14 R15** R16** R17** R18* [A12] [A13] [A14] [A15] [A16] [A17] [A18] [A19] [A20] [A21] R19-20** Page 380 of 411 380 [A22] [A23] [A24] [A25] [A26] [A27] [A28] [A29] [A30] [B1] [B2] [B3] [B4] How should multinodular thyroid glands. What are the best methods for long-term follow-up of patients with thyroid nodules? Neck imaging - Ultrasound R21-22 R23A-C** R23D* R24* R25-29 Thyroid Downloaded from online. Should a posttherapy scan be performed following remnant ablation or adjuvant therapy? What is the optimal directed approach to patients with suspected structural neck recurrence? Nodal Size Threshold Extent of Nodal Surgery Ethanol Injection* Radiofrequency or Laser Ablation* 382 131 R55-56** R57 R58 R59 Thyroid Downloaded from online. R60 R61 R62-63** R64 R65 R66-67 R68 R69* [C15] [C16] [C17] [C18] [C19] [C20] [C21] R70** T15* R71 Page 383 of 411 383 [C22] [C23] [C24] [C25] [C26] Thyroid Downloaded from online. How should patients who have received radioiodine therapy be monitored for risk of secondary malignancies?
When the mother is suffering from chronic conditions gastritis chronic nausea order clarithromycin now, and has to regularly take drugs chronic gastritis risk factors 250mg clarithromycin otc, and the doctor decides gastritis during pregnancy order clarithromycin 250mg online, if she can continue breast feeding or not gastritis symptoms fever purchase clarithromycin 500 mg on-line. In case she is allowed to continue to breast feed her baby, the baby should be closely monitored (observed) by the doctor for any possible harmful effects. It is important to remember that besides very few chronic conditions a mother is never advised to refrain from breast feeding. Advice on breast feeding varies as the drugs vary and is discussed in this column whenever necessary. A significant amount is found in breast milk - not known to be harmful but advisable to avoid using. It appears that iodine is concentrated in breast milk and can severely affect the thyroid gland of the baby. Nutritious food, cleanliness and vaccinations are three important bodyguards that protect children against many diseases. An important question which arises is that should children be given so many drugs for their illness? However, the fact remains that too many drugs are being given to infants and children although most of them have very little or no value. Besides, subjecting children to lot of drugs means subjecting them to lot of adverse effects. The main reason for children being more prone to adverse side-effects of drugs is that children are not just small adults. Hence if adult doses of a drug are given to children, drugs get accumulated in their body and produce harmful effects. This is why it is important that accurate doses be calculated for children taking into consideration both their age and weight. Certain drugs are harmful to children even in therapeutic doses and should be completely avoided, for example, loperamide, tetracycline. Advice on use of individual drugs in children is given in this column wherever necessary. Guidelines on the Use of Drugs in Children While administering drugs to children, particularly neonates (first 30 days of life), special care is always needed because they differ from adults in their response to drugs. Doses should invariably be calculated on the basis of weight till 50 kg or puberty is reached. In the neonatal period, the risk of toxicity is higher due to inefficient renal clearance, relative deficiencies of various enzymes, heightened sensitivity and inadequate detoxifying mechanism. It is always a good practice to state the age of child patient while writing prescriptions. Even though liquid preparations are more easily accepted by children, many contain sucrose which can lead to dental decay. Hence adjustment would need to be made for lower and upper limits of the stated range. Dose Calculation: the dosage for children can be calculated from adult doses by using either age, or body-weight or body surface area or by a combination of these factors. Even though body-surface area provides the most reliable method of determining dosage, in practice it is exceedingly difficult. Body-weight can be easily used to calculate doses and are generally expressed in mg/kg. Because of their higher metabolic rate, children generally require higher dose per kilogram than adults. This method can pose problems while calculating dose for obese children since they are liable to be given higher than required dose. Under such circumstances, it is better to calculate dose based on ideal body weight of the child in that particular age. Thus to calculate the dose for a child the following formula is used: Approximate dose for child = Surface area of child (m) X adult dose 1. In the case of children, some flexibility may be allowed so that they are not woken up at night. This is because health problems are more prevalent among the elderly as a result of which they use several different drugs at the same time. Besides, their liver and kidney (organs which break down and eliminate drugs from body respect), are not efficient enough and hence drugs get accumulated in their body producing harmful effects.