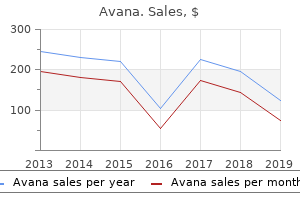
"Avana 50 mg overnight delivery, erectile dysfunction johnson city tn".
By: B. Emet, MD
Assistant Professor, Weill Cornell Medical College
Newborn screening program-The association of the Department erectile dysfunction drugs generic buy avana, the testing laboratory and the health care provider to ensure that every newborn child born in this Commonwealth has a blood specimen collected and screened for the newborn diseases in § 28 erectile dysfunction pills for heart patients discount avana online amex. Presumptive abnormal test result-An abnormal screening test result which is sufficiently abnormal to indicate the probable presence of a newborn disease listed in § 28 erectile dysfunction treatment nz discount 100 mg avana free shipping. Repeat specimen-A specimen collected from a newborn child on a specimen collection form after the initial specimen hypogonadism erectile dysfunction and type 2 diabetes mellitus buy generic avana 100mg on line. Specimen collection form-The official newborn screening program specimen form that includes both a multipart section for providing required information about the newborn child and a filter paper tab for application of blood. Testing laboratory-The licensed clinical laboratory under contract with the Department to perform testing for the newborn diseases listed in § 28. Transfer-The release of the newborn child from care and custody within and by a birth center or hospital and subsequent admission to another hospital. Treatment center-A center under contract with the Department to provide expert consultation, diagnosis and treatment for children with a presumptive abnormal test result. Unacceptable specimen-A blood specimen collected from a newborn child on a specimen collection form which is found to be unsuitable for testing in accordance with accepted laboratory testing standards as determined by the Department. The entry shall include a written statement of the objection signed by the parent or guardian. The Department will publish these standards, and any revisions thereto, in a notice in the Pennsylvania Bulletin. If the newborn child is transferred to another hospital for continuing care prior to 48 hours of age, the hospital to which the newborn child has been transferred shall collect a specimen from the newborn child, regardless of feeding history or medical condition, as close to 48 hours of age as possible but not later than 72 hours of age. If the newborn child is to undergo an exchange transfusion, the birth center or hospital shall collect the initial specimen for testing immediately prior to the exchange transfusion. If the newborn child is discharged from the birth center or hospital before 24 hours of age, the birth center or hospital shall collect the initial specimen from the newborn child as close to the time of discharge as is practicable, regardless of feeding history or medical condition. The birth center or hospital shall give the parent or guardian in whose care and custody the newborn child is discharged written notification of the need for a repeat specimen and shall also provide instructions to the parent or guardian for obtaining a repeat specimen from the newborn child as described in § 28. A health care practitioner who delivers a newborn child other than in a birth center or hospital shall collect or cause to be collected the initial specimen from the newborn child, regardless of feeding history or medical condition, as close to 48 hours as possible but not later than 72 hours of age. Within 72 hours of receipt of notice from the Department or testing laboratory, the health care provider that collected the initial specimen shall collect or cause to be collected from the newborn child a repeat specimen. The health care provider shall promptly notify a parent or guardian of the newborn child. When a sick child exhibits symptoms suggestive of a newborn disease listed in § 28. A health care provider offering maternity and newborn services shall collect and forward data semiannually to the Department on the number of patients for whom specimens for newborn disease testing have been collected and the number of patients for whom the specimens have not been collected, together with the reason in each instance for the failure to collect. No part of the information on this site may be reproduced for profit or sold for profit. This material has been drawn directly from the official Pennsylvania Code full text database. Appendix 6: Newborn Child Testing Act as ammended by Act 36 of 2008 2 Newborn Child Testing Act (as amended by Act 36 of 2008 effective 7/1/2009) 35 P § 621, et. Short title this act shall be known and may be cited as the "Newborn Child Testing Act. The department shall establish, by periodic publication in the Pennsylvania Bulletin, the method for reporting test results to the department. Procurement of specimens by health care providers (a) Health care providers shall cause to be procured blood specimens of newborn children for required screening and confirmatory tests and send such specimens to a testing laboratory designated by the department. Regulations the department, with the approval of the board, shall have the authority to promulgate regulations for the implementation and administration of this act. Community health district/county map of Pennsylvania Community health district/county map of Pennsylvania. Annually, an estimated 3,000 abnormal newborn screening results are identified for the six mandated conditions, resulting in approximately 200 diagnosed cases. Taken together, it is presumed that these pathomechanisms may underlie the neurological symptoms and brain abnormalities observed in the affected patients. Keywords: L-2-hydroxyglutaric aciduria; L-2-hydroxyglutaric acid; redox homeostasis; cerebral cortex; striatum; histopathology. To date, there are over three hundred documented cases of this disease in the medical literature [2-7]. The main neurological manifestations include progressive mental retardation, variable motor impairment and cerebellar ataxia.
Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S erectile dysfunction causes prescription drugs cheap 100 mg avana visa, International Committee on Thrombosis and Haemostasis erectile dysfunction protocol review scam buy cheap avana 200 mg line. Miletich J impotence 2 order 100mg avana, Sherman L female erectile dysfunction treatment purchase avana overnight delivery, Broze G Jr: Absence of thrombosis in subjects with heterozygous protein C deficiency. Congenital heterozygous protein C deficiency may predispose to thrombotic events, primarily venous thromboembolism. Protein C antigen and activities generally are undetectable in individuals with severe, homozygous protein C deficiency. Reference Values: Adults: 70%-150% Normal, full-term newborn infants or healthy premature infants may have decreased levels of protein C antigen (15%-50%), which may not reach adult levels until later in childhood or early adolescence. Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S, International Committee on Thrombosis and Haemeostasis. Miletrich J, Sherman L, Broze G Jr: Absence of thrombosis in subjects with heterozygous protein C deficiency. Congenital protein S deficiency is an autosomal codominant disorder that is present in 1% to 3% of patients with venous thromboembolism. Heterozygous protein S deficiency carriers have approximately a 10-fold increased risk of venous thromboembolism. Other phenotypic expressions of heterozygous congenital protein S deficiency include recurrent miscarriage, complications of pregnancy (preeclampsia, abruptio placentae, intrauterine growth restriction, and stillbirth) and possibly arterial thrombosis. Acquired deficiency of protein S is much more common than hereditary protein S deficiency and is generally of unknown hemostatic significance (ie, uncertain thrombosis risk). Acquired protein S deficiency is of uncertain clinical hemostatic significance and is associated with a variety of conditions. Reference Values: Males: 65-160% Females <50 years: 50-160% > or =50 years: 65-160% Newborn infants have normal or near-normal free protein S antigen (> or =50%), although total protein S antigen is usually below the adult reference range. There are insufficient data concerning protein S activity in normal neonates, infants, and children; but normal or near-normal activity (> or =50%) probably is present by age 3 to 6 months. Congenital protein S deficiency is an autosomal dominant disorder that is present in 2% to 6% of patients with venous thrombosis. Patients with protein S deficiency have an approximately 10-fold increased risk of venous thrombosis. In addition they may also experience recurrent miscarriage, complications of pregnancy (preeclampsia, abruptio placentae, intrauterine growth restriction, and stillbirth) and possibly arterial thrombosis. Three types of protein S deficiency have been described according to the levels of total protein S antigen, free protein S antigen, and protein S activity in plasma. Acquired deficiency of protein S has causes that are generally of unknown haemostatic significance (ie, uncertain thrombosis risk), and is much more common than hereditary protein S deficiency. Measurement of plasma free protein S antigen is performed as the initial testing for protein S deficiency. When the free protein S antigen level is below the age- and sex-adjusted normal range, reflexive testing will be performed for total plasma protein S antigen. Useful For: Investigation of patients with a history of thrombosis Interpretation: Protein S values vary widely in the normal population and are age- and sex-dependent. An increased total protein S antigen is of uncertain clinical significance because free protein S antigen levels are usually normal, in such situations. However, the total protein S antigen level may be helpful in distinguishing acquired versus congenital protein S deficiency. High normal or increased total protein S antigen and reduced free protein S antigen suggests acquired protein S deficiency, as may be seen in pregnancy or inflammation. Differentiation of congenital and acquired protein S deficiency requires clinical correlation and may require repeated laboratory study of the patient and selected family members in some instances. Grandrille S, Borgel D, Ireland H, et al: Protein S deficiency: a database of mutations. Wolf M, Boyer-Neumann C, Peynaud-Debayle E, et al: Clinical applications of a direct assay of free protein S antigen using monoclonal antibodies. Laroche P, Plassart V, Amiral J: Rapid quantitative latex immunoassays for diagnosis of thrombotic disorders. Increased amounts of protein in the urine may be due to: -Glomerular proteinuria: defects in permselectivity of the glomerular filtration barrier to plasma proteins (eg, glomerulonephritis or nephrotic syndrome) -Tubular proteinuria: incomplete tubular reabsorption of proteins (eg, interstitial nephritis) -Overflow proteinuria: increased plasma concentration of proteins that exceeds capacity for proximal tubular reabsorption (eg, multiple myeloma, myoglobinuria) -Urinary tract inflammation or tumor -Preeclampsia -Orthostatic proteinuria In pregnant women, a urinary protein excretion of >300 mg/24 hours is frequently cited as consistent with preeclampsia, and 12-hour total protein excretion highly correlates with 24-hour values in this patient population (1,2). Orthostatic proteinuria is characterized by increased protein excretion in the upright position, but normal levels when supine.
Purchase genuine avana. My erectile dysfunction is gone forever.
If bleeding is noted at the time of rupture of membranes erectile dysfunction injection therapy cost order avana with amex, vasa previa should also be considered erectile dysfunction before 30 effective 50 mg avana. Ultrasound findings erectile dysfunction exercise video effective avana 200mg, if present erectile dysfunction pills dischem discount avana 200 mg visa, may include a retroplacental echolucency, abnormal thickening of the placenta, or an abnormally round torn up edge of the placenta. A Cochrane review found no randomized controlled trials assessing interventions for placental abruption that met inclusion criteria. They propose medical induction at 37 weeks in women with a history of placental abruption. Women experiencing recurrent bleeding attributed to placental separation may be diagnosed as having a chronic abruption. When expectant management occurs in the setting of chronic abruption, serial ultrasonography for fetal growth and antepartum surveillance are indicated in the third trimester because of the potential for uteroplacental insufficiency. However, acute blood clots and the placenta are hyperechoic on ultrasonography and can be difficult to distinguish from one another. If the mother and fetus are stable, the placental location and appearance and fetal lie and fetal weight estimation Severe Abruption Initial management includes rapid stabilization of maternal cardiopulmonary status and assessment of fetal well-being. Delay can be fatal to the fetus; 30% of perinatal mortalities in one case series occurred within 2 hours of admission. In patients with preeclampsia or other confounding factors, central blood pressure monitoring may assist in the fluid management. Neonatal resuscitation personnel should be available for all deliveries, vaginal or operative. When fetal mortality occurs secondary to abruption, vaginal delivery should be the goal. Oxytocin augmentation is not contraindicated, but should be used judiciously with intrauterine pressure monitoring. Indications for operative delivery with fetal demise include other maternal indications for cesarean delivery, failure of labor progression, and brisk hemorrhage that cannot be compensated for by transfusion. Approximately one-third of patients with placental abruption with fetal demise will develop coagulopathy. Coagulopathy is typically not seen in the patient presenting with abruption and a live fetus. Replacement of platelets and fresh-frozen plasma should be administered just before operative delivery to provide maximum efficacy. Patients presenting with abruption and a live fetus are typically not stable for transfer because operative delivery may be needed on an immediate basis at any time during labor. In this instance, neonatal transfer (rather than maternal-fetal) may be a necessary intervention for the premature or sick newborn. If fetal demise has occurred, a patient who does not have coagulopathy and is hemodynamically stable may be cared for with appropriate resources. Blood bank supply may determine whether or not a patient needs transport to a referral facility. The KleihauerBetke test is useful to determine dosage of Rho (D) immune globulin in Rh-negative patients, but is not useful for the diagnosis of abruption. In uncommon instances, uterine rupture can be spontaneous and occur in the absence of risk factors. In complete rupture, the fetus or placenta may be partially or completely extruded from the uterus. This chapter focuses only on uterine rupture presenting with third trimester bleeding. Classic or T-shaped uterine incisions are associated with a higher likelihood of uterine rupture compared with a low transverse incision. In a case series of women experiencing spontaneous uterine rupture in the second or third trimester, six of seven events (during 13 years) involved placenta previa or percreta and five of the seven uterine ruptures occurred in women with prior cesarean deliveries. If these measures are not effective, then emergent cesarean or operative vaginal delivery may be indicated. Asymptomatic scar disruption may be found at the time of cesarean delivery or palpation of the uterine cavity after vaginal delivery. Routine inspection of the uterine scar after vaginal delivery is not recommended and asymptomatic scar disruption is expectantly managed. Clinical Presentation the classic presentation for symptomatic, significant uterine rupture includes vaginal bleeding, pain, cessation of contractions, absence of fetal heart tones, loss of station, easily palpable fetal parts through the maternal abdomen, and profound maternal tachycardia and hypotension. However, most cases (67% to 70%) of uterine rupture initially present with abnormal fetal monitoring.
However erectile dysfunction medicine in uae buy avana 100mg line, the physiologic role of Grb proteins is not fully clear erectile dysfunction treatment germany generic 200mg avana otc, and their actions may be tissue specific with capabilities as an inhibitory factor or a positive mediator in the insulin signaling pathway erectile dysfunction and smoking purchase 200 mg avana fast delivery. Several of these kinases have been shown to function as physiologic modulators causing desensitization of insulin signaling pathways under conditions of nutrient excess erectile dysfunction kidney order avana from india, inflammation and cell stress responses. This raises the possibility that the cytokine could be inducing cellular insulin resistance via autocrine and/or paracrine effects. In fact, treatment with high doses of salicylates improves glucose tolerance and enhances insulin sensitivity in humans and rodents [59]. Evidence suggests that a similar mechanism may be operative for the insulin receptor [46]. Tissue-specific insulin action: the role of insulin effector systems Insulin regulates whole-body fuel homeostasis via specific effects in multiple target tissues. The nature of these biologic actions varies dramatically from tissue to tissue, and these variations, for the most part, are not brought about by differences in insulin signal transmission (described above). Rather, tissue-specific insulin effects are principally explained by effector systems that are uniquely expressed in a variety of differentiated target cells. The biochemical basis of these effects is described in skeletal muscle, adipose tissue, and liver, three organs primarily responsible for fuel storage and oxidation as well as counter-regulatory metabolism. Skeletal muscle Insulin stimulation of glucose transport Skeletal muscle accounts for the bulk of insulin-stimulated glucose uptake in vivo, and the hallmark of insulin action in this tissue is the ability to stimulate the glucose transport effector system (Figure 7. Each glucose transporter isoform has a specific role in glucose metabolism determined by its pattern of tissue expression, substrate specificity and affinity, transport kinetics, and regulated expression in different physiologic conditions. Regarding actin, insulin stimulates cytoskeletal rearrangement with the appearance of cortical -actin fiber projections that subtend the plasma membrane, and this actin remodeling is under the control of small G-proteins in the Rho, Rab and Rac families. The complete pathway linking insulin signal transduction to stimulation of the glucose transport system has not been fully elucidated. These observations indicate that signaling systems mediating glucose transport stimulation are different in response to acute exercise versus insulin [72]. Adipose tissue Adipose tissue is the predominant site for fuel storage as triglyceride, and effector systems responsible for the anabolic effects of insulin on lipogenesis and antilipolysis are key aspects of adipocyte biology (Figure 7. Lipogenesis Fat accumulation in adipocytes is determined by the balance between triglyceride synthesis (fatty acid uptake and lipogenesis) and breakdown (lipolysis/fatty acid oxidation). Insulin augments availability of both glycerol and fatty acids for triglyceride synthesis by increasing the uptake of glucose in the adipose cell as well as by activating lipogenic and glycolytic enzymes. These enzymes constitute the effector system for the biologic effects of insulin on lipogenesis, and are modulated by insulin both through post-translational modifications and alteration of gene expression. Arrows represent an activation process; blocked arrows represent an inhibition process. It activates a battery of genes involved in the uptake and synthesis of fatty acids and triacylglycerides. Perilipins are localized at the surface of the lipid droplet in adipocytes [79], and are essential in the regulation of triglyceride deposition and mobilization. In the absence of lipolytic stimulation, perilipin inhibits lipolysis by acting as a barrier against hydrolysis of the triacylglycerol by lipases. In adipocytes there are two forms of perilipin, perilipin A and perilipin B, with perilipin A present at a higher concentration. Adipocyte perilipin content has an inverse correlation with lipolytic rates and a positive correlation with plasma glycerol in humans, and is reduced in obese women. The rise (after a meal) and fall (with fasting) of insulin has a central role in this regulatory process as a result of its antilipolytic action in adipocytes. Lipolysis in normal subjects is exquisitely sensitive to inhibition by insulin, such that half-maximal suppression of lipolysis occurs at insulin concentrations well below those needed for significant stimulation of glucose uptake by skeletal muscle. The stimulation of glycogen formation and regulation of gluconeogenesis by insulin are the critical determinants of hepatic glucose output. In addition, because regulation of gene transcription is critical for the biologic effects of insulin on hepatic metabolism, mechanisms pertinent to transcriptional regulation are discussed (Figure 7. Glycogenesis/glycogenolysis Insulin exerts dramatic effects on pathways of intracellular glucose metabolism. Under conditions of insulin stimulation, the major portion of glucose uptake is stored as glycogen in humans. Insulin promotes glycogen synthesis in muscle, adipocytes, and liver by activating glycogen synthase, which adds activated glucosyl groups to growing polysaccharide chains and thus catalyzes the final step in glycogen synthesis.