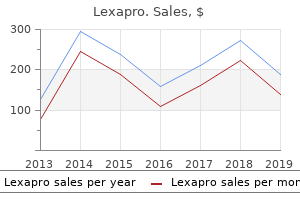
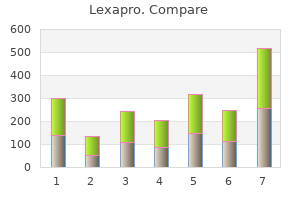
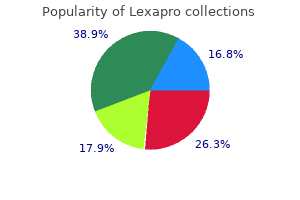
"Discount lexapro 10mg mastercard, anxiety urban dictionary".
By: W. Dolok, M.A.S., M.D.
Co-Director, Ponce School of Medicine

These data are consistent with a transient and reversible fusion of secretory granule and plasma membranes anxiety 0-10 scale purchase lexapro. A third line of evidence supporting the idea of a fusion pore is that the current that flows through the fusion pore as the secretory-granule membrane discharges its membrane potential depression glass defined purchase lexapro 5 mg without a prescription. From measurements of the conductance of the channel bipolar depression medicines lexapro 20mg without a prescription, estimates of its size can be made depression lies buy lexapro 10 mg lowest price. When it first forms, the fusion pore seems to form a channel of about the size of a large ion channel. Fusion between a membrane-covered virus and the plasma membrane has been studied extensively for influenza virus. The viral protein triggers fusion by undergoing a conformational change that extends a 16- to 23-amino acid residue "fusion peptide," a hydrophobic sequence that is essential for fusion (44). Extension of the fusion peptide requires a helical hairpin to be converted to a triple-stranded coiled-coil domain (45). Nor is it clear whether the initial hole is exclusively due to rearrangement of the lipid bilayer or to a proteinaceous channel. In influenza virus-mediated fusion, a hemifusion state is formed if the transmembrane tail of the fusion protein is replaced by a glycophosphatidylinositol anchor (46). This was interpreted to mean that the hemifusion state is an intermediate in virus-induced fusion. Logically, membrane fusion must be initiated by the formation of one or several small holes through the membrane of both the exocytotic vesicle and the cell surface. Electrophophysiologically, the evidence for the reversible formation of such a hole is excellent. Exon Shuffling When intervening sequences were discovered to split eukaryotic genes into segments coding for protein, the intervening sequences were named introns and the coding regions, exons (1) (see Introns/exons). Since then, heated discussion has ensued concerning the evolutionary origin and biological significance of introns in eukaryotes, because almost all of the prokaryotic genes lack introns, and the introns have no known role. Gilbert (1) proposed a scenario in which introns simply connect neighboring exons to each other in the genome; consequently, genetic recombination would not harm the coding portions of genes if it took place within the intron regions. Such recombination within introns would facilitate the shuffling of exons, to create new exon combinations and lead to the emergence of new genes with new functions. Exon shuffling is related to the present controversy regarding the "early" versus "late" intron theories. The early-intron theory maintains that introns existed in the ancestor genomes of prokaryotes and eukaryotes, but that all those in prokaryotes were deleted by some unknown mechanism. On the other hand, the late-intron theory contends that introns could have been inserted, similar to transposable elements, into eukaryotic genomes quite recently, at least after the divergence of eukaryotes and prokaryotes. This theory is based on the observation that there are many genes in which the locations of introns are not conserved among vertebrates, invertebrates, and plants (2). If the early-intron theory is correct, exon shuffling probably had a significant role in the creation of new genes. If the late-intron theory is right, however, exon shuffling might have been significant only after the introns were inserted. The domain shuffling theory is similar to the exon shuffling theory, but in sharp contrast, maintains that the unit of shuffling during evolution is a functional protein domain, not an exon. Although there are some cases where it does, most functional domains consist of more than one exon or occupy only part of a large exon. For example, the Kringle domain is known to have been shuffled as a unit during evolution. The Kringle domain is a characteristic supersecondary structure frequently found in the serine proteinases involved in the blood clotting system. Even though the Kringle domain is, in most cases, split into three exons by two introns, those found in various proteins are almost always complete forms and never found as parts corresponding to the exons. At present, however, there is no known genetic mechanism for facilitating domain shuffling, and domain shuffling seems to have taken place in prokaryotes as well (3). Expansibility the expansion of a protein molecule with increasing temperature reflects its atomic packing and flexibility, as does its compressibility with increasing pressure.
So an individual of group A will make anti-B alloantibodies depression symptoms nhs discount lexapro 5mg mastercard, an individual of group B will produce anti-A mood disorder social security disability buy lexapro 5mg line, and one of group O will have both mood disorder 504 plan purchase 20 mg lexapro otc. Very severe accidents in blood transfusion would result from the agglutination of the donor red blood cells by the alloantibodies of the recipient depression youtube lexapro 10 mg cheap. The reverse situation, agglutination of the host red blood cells by antibodies of an incompatible donor, should also be avoided, although accidents are less severe. Even if the genetic determinism of these alloantigens is straightforward, it still is not clear why the alloantibodies corresponding to the nonexpressed substance(s) are produced. The prevalent explanation is that blood groups cross-react with bacteria normally present in the intestinal flora, which would stimulate the immune system to produce the corresponding crossreacting antibodies. It should be stressed that these "natural" antibodies are of the IgM type, which may be directly related to the fact that they are the result of the stimulation by a T-independent polysaccharide antigen. The titer of alloantibodies varies greatly between individuals, but may be considerably elevated upon an incompatible transfusion. Another famous case of alloantigen in the blood groups is the Rhesus factor, which was responsible for the dramatic hemolytic disease of the newborn, due to the immunization of an Rh mother against red blood cells of an Rh+ fetus. This occurs during the delivery because some fetal red cells may enter the maternal circulation and induce the formation of IgG antibodies that will actively cross the placental barrier in a subsequent pregnancy and then provoke lysis of Rh+ fetus red cells. It has now been generalized to prevent the immunization by injecting the Rh mother with anti-D (anti-Rhesus) antibodies immediately after delivery, to trap the red blood cells from the newborn that would have penetrated the maternal circulation at birth. Many other alloantigens are known, but alloantibodies are generally not produced unless the antigen is given. The name of major histocompatibility complex indicates by itself how these molecules were first discovered as a major target for graft rejection, as the result of a very severe alloimmune response, characterized primarily by the production of cytotoxic T lymphocytes. Many other systems may behave as potential alloantigens, which simply reflects the existence of allelic variants. The case of allotypes of immunoglobulins has been studied particularly by immunologists and has provided remarkable genetic markers for the study of immunoglobulin biosynthesis and diversity at the time. Allophenic Allophenic individuals are composed of cells of two different genotypes (often called mosaics or chimeras). Allophenic mice are the basis of the revolution in mouse genetics, allowing mice with specific gene replacements to be generated and studied. Allophenic mice are made by mixing cells from two embryos of different genotypes (1). These new composite embryos are implanted into foster mothers and allowed to develop. The early embryos are able to compensate and form a single individual from the mixture of embryonic cells. The mosaic progeny that result from these manipulations have tissues that contain cells of the two different genotypes. When the germ cells are also of mixed origin, progeny can be recovered from both genotypes. The ability to make allophenic mice in the laboratory was first used to study the contributions of cells to individual tissues. The number of cells that give rise to a particular tissue can be estimated from the proportions of the two genotypes in a large number of allophenic mice. The cellular autonomy of mutant phenotypes can be determined by generating allophenic mice between mutant and wild-type mouse embryos. The two cell types usually differ both in the mutant of interest and in some marker genotype, such as an enzyme polymorphism. Two advances in the technology of allophenic mice have contributed to a revolution in mouse genetics in the last few years. The first advance was the ability to use embryonic teratocarcinoma cells from cell culture as one of the two cell types used to make the allophenic mice (2). The cultured cells are injected into the interior of a normal mouse embryo and are incorporated into the embryo, to form an allophenic mouse. The teratocarcinoma cells are totipotent and can even form germ cells that give rise to the gametes for the next generation. The second major advance was the development of techniques for site-directed mutagenesis and gene replacement in mammalian cells in culture (3) (see Gene Targeting). A gene of interest is altered in a specific way in teratocarcinoma cells in culture. A clone of cells with the altered genotype is produced, and cells from the clone are injected into early mouse embryos to produce allophenic mice.
Generally depression fallout order 10mg lexapro with visa, membranes made with "pure" cellulose nitrate are preferred for most techniques in molecular biology depression definition dsm 5 cheap 5 mg lexapro overnight delivery, because "pure" cellulose nitrate has a higher binding capacity anxiety 504 accommodations purchase 10mg lexapro with mastercard. Nitrocellulose membranes are widely used in molecular biology because they are easy to handle emotional depression test buy discount lexapro on-line, have a high binding capacity, and are compatible with a variety of assay conditions and detection systems. Although nitrocellulose membranes were originally used to filter out particles, such as bacteria, they are now used primarily to adsorb macromolecules throughout the filter matrix, as in filter binding assays and in blotting. Procedures that frequently specify nitrocellulose membranes include: Southern blots and Northern blots; nucleic acid and protein dot-blots and slot-blots; immunoblots; and colony/plaque lifts. Nitrocellulose membranes are used in Western blots of proteins, but they cannot be used for Edman Degradation sequencing, because the membranes will dissolve in the sequencing solvents. How macromolecules bind to nitrocellulose is not well understood, but both electrostatic and hydrophobic interactions have been suggested as possible binding mechanisms (2, 3). Additional support for the hypothesis that hydrophobic interactions play a dominant role in the binding of proteins to nitrocellulose membranes comes from a study that compared the binding of antibodies and other proteins to nitrocellulose membranes in acidic, neutral, basic, and chaotropic buffers (7). In this study, radiolabeled proteins of various molecular weights and isoelectric points bound equally well to nitrocellulose in a variety of buffers (at pH 2, 3, 7, 12, and 13) and in the presence of 8 M urea (at pH 2, 3, and 7) and 6 M guanidinium chloride. This study demonstrated that the binding of most proteins to nitrocellulose was not influenced by the ionization of the proteins or by denaturing solvents. Binding of 125I-labeled human anti-tetanus antibodies to a nitrocellulose membrane in different solvents. The indicated buffers and denaturing solvents were used for both membrane equilibration and protein dilution. After binding the antibodies to the membrane, each was washed four times with 200 µL of 0. The nitrocellulose membrane was removed from the dot-blot unit, and the areas that contained the radioactive proteins were counted in a gamma counter. Vapors of some organic solvents may cause the nitrocellulose to curl, and both oil and dust may prevent binding of macromolecules. Wet a nitrocellulose membrane by floating, not immersing, the membrane on the top of water to remove air that may be trapped within the matrix. Air bubbles trapped under the membrane can be removed by using tweezers to lift a corner. After wetting, immerse the membrane in the appropriate buffer for at least five minutes. Wet nitrocellulose membrane will have a uniform darkening, and any white spots or irregularities are signs of incomplete wetting. Verify that the macromolecule of interest binds to the nitrocellulose membrane and is not eluted by the detergents or other solutions used in the assay. Solutions containing methanol or ethanol may cause the nitrocellulose to shrink, and acetone will dissolve it. However, xylene and toluene can be used to make the nitrocellulose transparent for scanning with a transmission densitometer (8). Do not exceed 80°C in the vacuum baking oven when drying nucleic acid blots, because nitrocellulose is quite flammable. Unsupported nitrocellulose membranes are brittle when dried or baked at 80°C, so handle them very carefully! Gershoni (1991) "Immunoblotting: membrane filters as the solid phase for immunoassays". Because ammonia is necessary for the formation of biologically essential, nitrogen-containing compounds, such as amino acids and nucleic acids, a fixed nitrogen source is necessary to sustain life on earth. Furthermore, the ammonia necessary to support essential biosynthetic reactions is continually sequestered into sediments or reconverted to N2 through the combined biological processes of nitrification and denitrification. So the pool of fixed nitrogen within the biosphere must constantly be replenished. Nitrogen fixation is necessary to maintain the diversity of life on earth because most organisms cannot metabolize the abundant but relatively inert N2 molecule and must assimilate nitrogen in a "fixed" form, such as ammonia or nitrate. The three ways that nitrogen fixation occurs in the biosphere include (1) lightning and other natural combustion processes, (2) the industrial HaberBosch process, and (3) biological nitrogen fixation. Of these three, biological nitrogen fixation, the most significant contributor, accounts for about 65% of the total (1, 2).
Morris (1986) Nuclear Magnetic Resonance Imaging in Medicine and Biology mood disorder webmd order lexapro 10 mg fast delivery, Clarendon Press anxiety of influence cheap lexapro 20 mg visa, New York bipolar depression definition purchase discount lexapro. In it tropical depression definition wikipedia purchase lexapro with american express, cross peaks appear because the spins of atoms are sufficiently close to each other that their mutual interaction, through relaxation, can produce changes in the populations of the nuclear spin energy levels that are associated with those spins. The chemical-shift coordinates of a cross peak identify the shifts of the interacting sets of spins. The distance dependence of the cross-peak intensity is very great, in the simplest case varying with 1/r 6, where r is the distance between the spins. Spin diffusion is a time-dependent phenomenon, so the misleading cross peaks it produces can be minimized by reducing the mixing time. The heights of all cross peaks are altered to some extent by this procedure, however, and all cross-peak intensities may be perilously close to experimental noise if the mixing time is short. Williamson (1989) the Nuclear Overhauser Effect in Structural and Conformational Analysis, V. It encodes a secreted glycoprotein that is a disulfide-linked dimer in solution (3). Noggin gained its name from its ability to transform the entire embryo into head structures. The possible zygotic function of noggin was tested by applying Noggin recombinant protein to explants. When applied to prospective ventral mesoderm or epidermis, Noggin can mimic two of the known gastrula-stage activities of the organizer, namely, dorsalization of ventral mesoderm and neural induction (3, 5). Neural induction by Noggin appears to be direct, in that no mesodermal intermediate is detected. Whereas mesoderm inducers can act only on blastula and early gastrula tissues, Noggin can induce neural tissue until late gastrulation. Although the treated explants are not homogeneous, only anterior types of tissue are present (5). Expression of the mouse noggin gene is homologous to that of Xenopus in that noggin transcripts are found in the mouse organizer (node) and its axial mesodermal derivatives. In situ hybridization and a lacZ reporter gene, integrated into and replacing the endogenous noggin-coding sequences, were used to detect later sites of noggin expression; these appear to be crucial (2, 8). Although the initial steps of neural induction and somite formation occur in a Noggin-deficient mouse, neural patterning and skeletal formation are abnormal. Thus, there is an increasing severity of ventralization in the neural tube, with the loss of interneurons, motor neurons, and finally the floor plate. These defects are mirrored in the mesoderm, where a rostral to caudal gradient of defects in the somites includes loss of sclerotomal and dermomyotomal derivatives. Nonautonomous Controlling Element "Controlling elements" was the term Barbara McClintock gave to transposable elements when she discovered them in maize in the late 1940s. This designation emphasized that these new genetic elements could control gene expression, as well as move from place to place in the genome (1). McClintock discovered two classes of controlling elements, one of which she designated Dissociation (Ds) and the other activator (Ac). She found that while Ds elements could move in the presence of Ac, they were unable to move alone, while Ac was capable of movement in the presence or absence of Ds. She hypothesized that activator made a product that could promote the movement of both Ac and Ds. We now know that this product is the Ac transposase and that Ds is a deletion derivative of Ac that contains the same special recombination sequences at its tips that the Ac transposase can act on, but is lacking a transposase. Because Ds lacks a transposase gene, it cannot promote its own movement and is dependent on this product from Ac. Different nonautonomous versions of the same element result from different internal deletions. These deleted versions probably arise from incomplete repair events at the donor site after transposon excision (2-4); this repair involves transferring information from an intact homologue, using homologous recombination and gap repair. In addition to AcDs and several other sets of autonomous and nonautonomous elements in maize, autonomous and nonautonomous elements have been also observed in other organisms such as Drosophila (2), and Caenorhabditis elegans (3, 5).
Discount lexapro online. You are NOT Alone - Depression Anxiety and Suicide Awareness | Kayla Stoecklein.